Your Cart is Empty






Lumiscope Portable Ultrasonic Nebulizer
F-6700
$115.99 - $149.99
- ▼The Details
-
- Adjustable flow volume and fast flow rate of up to 0.7 ml/min
- Extremely quiet operation
- Ultrasonic nebulization
- Comes with everything you need for an effective nebulizer treatment!
- Two-valve system ensures maximum efficiency of inhalation, reducing waste of medicine
- ◄What's in the Box
-
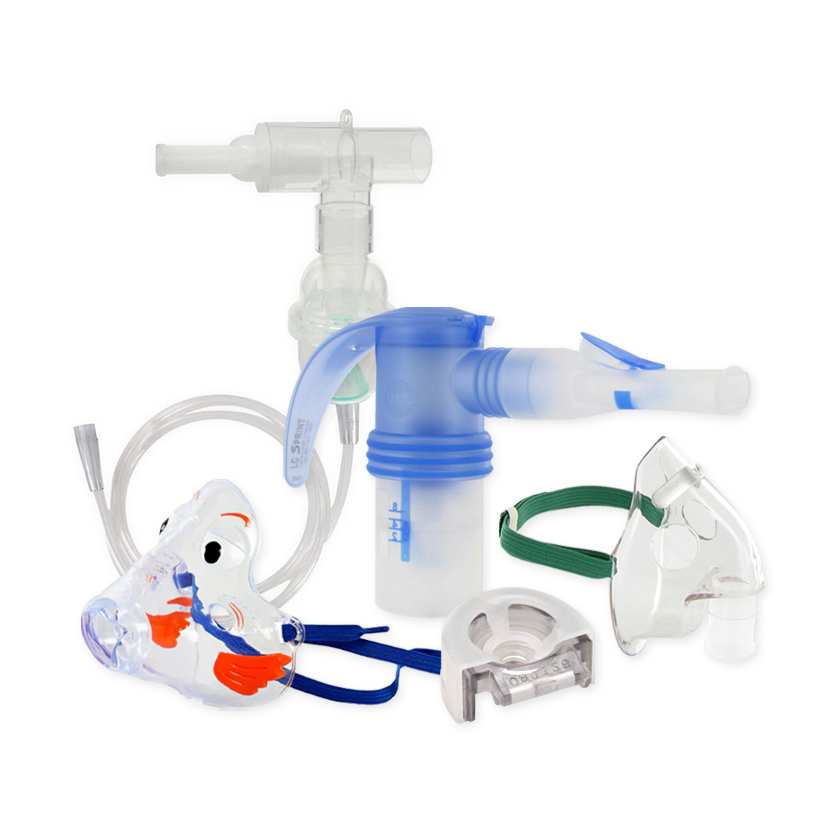
- Ultrasonic nebulizer
- Adult mask
- Pediatric mask
- 20 medication cups
- 5 replacement filters
- Mouthpiece
- AC adapter
- Optional rechargeable battery
- Carrying case.
- ◄Specifications
-
Attribute Details PRODUCT DIMENSIONS 5.1 x 3.3 x 2.2 in. (130 x 85 x 55 mm) WARRANTY One year limited warranty against defects in workmanship and materials. Warranty does not cover batteries. MANUFACTURER Graham-Field MANUFACTURER ITEM CODE 6700 METHOD OF NEBULIZATION Ultrasonic Technology PRESCRIPTION Required PRODUCT WEIGHT .44 Lb (w/o battery) PARTICLE SIZE 0.5~4.0 Microns 
- ◄Videos
- ◄Additional Info
-
View Owner's Guide
Portable Ultrasonic Nebulizer is designed to be used by an adult or pediatric patient to produce medicated aerosols for inhalation therapy. It features a two-valve system that ensures maximum efficiency of inhalation, thus reducing the waste of medication. In practice, the mouthpiece expiration valve will open during expiration, allowing the patient's exhaled air to pass to the atmosphere, avoiding contamination of the device. During inspiration, the valve will close, maximizing the inhalation amount. As with all ultrasonic nebulizers, this nebulizer is not for use with Pulmicort.
You have the option to purchase this ultrasonic nebulizer with or without a rechargeable battery. With the rechargeable battery you'll have the opportunity to take treatments nearly anywhere!
It's important to regularly replace this ultrasonic nebulizer's air filter every 30 days or when it appears dirty or gray. Not regularly replacing the air filter will cause your nebulizer to under perform, and cause treatments to be less effective. In addition, this unit requires distilled water: Fill the reservoir of the device with distilled water until it reaches exactly the level marked in red (approximately 4 ml). This amount of water serves as a liquid conductor, to conduct the ultrasound waves to the medication, and will never be nebulized.
To prevent possible risk of infection from contaminated medication, clean the nebulizer as follows after each treatment.
To clean: Ensure the plug is disconnected from the device's power socket. Remove the top cover, nebulization chamber, airflow chamber, and medication from the devise. Remove the filter from the cover. Completely empty the conduction liquid from the reservoir. Wipe the main unit with a clean, soft, moistened cloth. Wash the top cover, nebulization chamber, air-flow chamber, mouthpiece, masks, and medication cup in warm, soapy water, and rinse thoroughly. Allow to air dry. Store in a clean, dry location.
Once daily, after washing as above, soak the washed components in a fresh solution consisting of one part warm water and one part white vinegar for 30 minutes followed by a warm water rinse. Allow to air dry. Store in a clean, dry location.
- ◄Replacement Parts & Accessories
-